Archer Daniels Midland Co. (ADM) expects to see significant improvement in efficiency when it completes the commissioning of its new feed manufacturing facility in Quincy, Illinois, U.S.
The facility, which features three milling lines, brings together into one building capacity that is currently spread across five mills on the east and west sides of the street that occupies a significant footprint. Along with efficiency, the consolidation will mean improved safety for the feed produced and the people who produce it.
“When we talk about this facility and the why, there are three things,” said Ryan Goldie, director of manufacturing for ADM Animal Nutrition. “One is feed safety. We can simplify our process and eliminate some of that risk through complete segregation. There’s also human safety. Some of that facility was built back in the 1920s, so we’re dealing with all wooden structures and different electrical standards. And efficiency. We’re operating on 20 acres on both sides of 30th street. You lose a lot of efficiency gains when you’re trying to move product.”
The new facility, located on the same land as the current plant, has three independent lines: medicated feed, non-medicated feed and mineral, for a total capacity of 120,000 tons per year. In addition to the feed lines, the new facility has expanded warehouse space, automated packing and process controls and pressed tub production. When fully functional, it will turn 350 raw ingredients into 1,200 finished products for multiple species.
ADM initially purchased the Quincy facility from Moorman Manufacturing Co. in 1998. It is one of six ADM facilities in Quincy, where the company employs 500 people, including 200 at the feed facility.
Initial demolition work of flat storage and site preparation started in January 2017 but plans for the facility were in the works for at least five years, Goldie said. The concrete slipform building went up in September of that year. This is the fourth animal nutrition manufacturing location ADM has opened in North America since 2016.
Commissioning of the facility started in May and was expected to take 120 days with the plant fully operational in about six months, Goldie said.
“We have three independent milling lines in the facility so it’s a little bit of a process,” he said. “We’re going to do the medicated line first, and systemically go through each line from there.”
Improving safety and efficiency

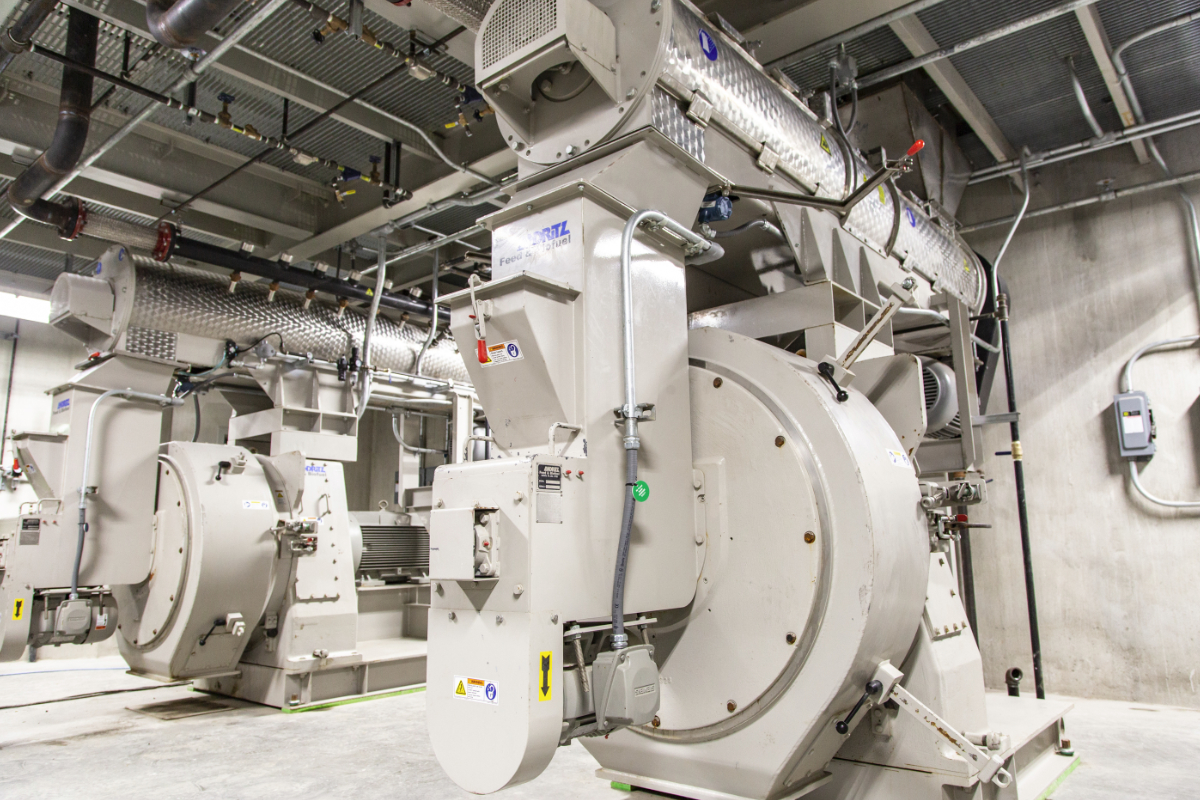
The Quincy mill has several unique features, the first being three separate milling lines under one roof. That presents challenges in startup but also will mean improved efficiency and safety.
“The big thing is separation, so we don’t have cross contamination among high-risk ingredients, which means we’re making it safer,” Goldie said.
The non-medicated feed line is completely separate, with its own mixer, pellet mill, weigh-lorry and bagging line.
There are interlocks built into the automation that prevent any type of cross contamination or prevent the wrong ingredient from getting into a formula, said Nathan Fry, plant manager.
“Automation takes the human error out of it,” he said. “We can use automation to ensure everything is happening in the right process and we don’t have cross contamination.”
The feed formula comes right out of the system and into the automation system supplied by BetaRaven, St. Charles, Missouri, U.S., which ensures the proper ingredients are added.
“We’re trying to eliminate the number of hand touches at the mixer,” Goldie said.
Ingredients that do have to be added by hand, such as medication or expensive ingredients needed in only small amounts, are bar coded to match the batch ticket. The bar code is scanned with the computer next to the locked hand-add location, which only will open if the correct ingredient has been scanned.
“There are a lot of steps in place to make sure we have the safest product going out the door,” Goldie said. “A little bit of the wrong ingredient in the wrong mix can be detrimental to the animal, the customer and obviously to our reputation.”
In terms of efficiency, the new facility drastically cuts down on the distance finished pellets need to be conveyed, from about 1,780 feet among the old facilities to 80 feet now.
“There’s a lot less horizontal movement of the pellets,” Fry said. “In today’s plant, they can travel through quite a few conveyors, which can have detrimental effects on the quality. In the new plant, everything is vertical so there’s very little movement of the pellets. Once it’s pelleted and cooled, it doesn’t go very far before it’s bagged. That produces a lot better, more consistent pellet.”
In the old facilities, a new mix couldn’t be started until the current mix had been conveyed out. This was done by visual inspection. In the new facility, the automation system will determine when a new mix can begin.
More product will be produced with fewer pellet mills at the new facility. Currently, four pellet mills produce about 9 to 10 tons per hour each. In the new plant, three pellet mills will be able to produce 15 to 20 tons per hour each, Fry said.
Labor will be used more efficiently as well, Goldie said. Instead of needing multiple people to run each mixer in the separate facilities, one employee will be able to operate the mixers at the new facility.
“The labor is used in different ways so it’s not a drastic cut in labor,” he said. “It’s used differently and a lot more efficiently.”
The Quincy facility is also unique in that it’s the only animal nutrition plant that produces a pressed tub product, Goldie said. All the bulk raw ingredients are combined and run through a six-ton mixer before being pressed into whatever the requirements are per tub based on pounds per square inch. It can produce a 100-pound up to a 250-pound tub depending on the species and the product.
Unlike most feed facilities, the Quincy plant is concrete. It took eight days to complete the slipform eight-story building. Younglove Construction, Sioux City, Iowa, U.S., served as the general contractor on the Quincy project.
“Beta Raven and Younglove work together well,” Goldie said. “Trying to pick the right partners on the construction and build side are pretty important.”
While there’s room and property available to build another mill, Goldie said they tried to size the new mill looking several decades ahead.
“We looked at what we’re doing today and what the market is going to look like in 20 years, and built for that,” he said. “The medicated line has two pellet mills and we used quick change dies for efficiency. We tried to be as agile as we could and designed so we can meet the capacity demand and customer quality for any changes that are going to come down the road.”
There’s plenty of room in the mill, so if five years down the road a piece of equipment needs to be added, there’s room to do so, he said.
Process flow

All bulk products arrive into the new plant by truck and enter the facility through one of two receiving pits with a capacity of 200 tph. The bulk products are sent to 40 different bulk bins. The facility also has totes or 2,000-pound super sacks. Each line has eight tote hangers for a total of 24.
Some of the raw commodities are sourced locally while others are sourced throughout the Midwest, depending on the ingredient metric, Goldie said.
The mineral line and the medicated line each have 24 micro bins and the non-medicated line has 12 micro bins, which can weigh ingredients out to the nearest tenth of a pound.
The Beta Raven automation system will run through the formula and pull the ingredients into separate mixers from Scott Equipment Co., New Prague, Minnesota, U.S., for the medicated and non-medicated lines. For the mineral line, the mixers are stainless steel because of the abrasive nature of the material. The mineral line includes a pug mill where liquids can be added to coat the minerals and a mix-muller from Simpson Technologies, Aurora, Illinois, U.S., that handles the raw ingredients for the pressed tubs. After mixing, pelleted feeds go through the pellet mills — two on the medicated line and one on the non-medicated line — supplied by ANDRITZ Feed and Biofuel, Muncy, Pennsylvania, U.S. Each pellet mill has a cooler from Bliss Industries, Ponca City, Oklahoma, U.S.
From the pellet mills, product goes into one of three bagging lines, supplied by Concetti, Bastia Umbra, Italy, and Premier Tech, Québec, Canada, or out to bulk bins. The facility has 32 bulk bins for loadout, three are non-medicated with a dedicated weigh-lorry that only goes to those three bins.
“It helps guarantee the equine customer that it’s mechanically impossible to get cross contamination into their feed,” Goldie said. “That’s a big selling point of the process for us.”
The facility will produce feed for multiple species, including cattle, swine, equine and poultry along with range mineral supplements, pressed tubs and weatherized loose granule minerals. Finished product, either bulk, bagged or in tubs, is transported by truck all over the United States. Certain products go to other commercial premix feed facilities across the country and it also services local customers within a 450-mile radius.
Feed expansion
ADM Animal Nutrition’s North American headquarters are in Quincy, adjacent to the new feed mill. The division operates 31 feed plants, six premix plants, four liquid feed plants, two agriculture centers, two blending stations, five warehouses, two specialty facilities and one research farm.
In 2016, ADM opened a high-tech feed facility in Glencoe, Minnesota, U.S., with a capacity of 100,000 tons and more than 30,000-square-feet of warehouse space. A new premix facility was built in Effingham, Illinois, U.S., and opened in spring 2018. Just a few months later, it opened a new feed manufacturing facility in Columbus, Nebraska, U.S., next to the existing mill that was built in 1968.
Along with the four new facilities, ADM has expanded its animal nutrition footprint. This year, it completed the acquisition of Neovia, a global leader in value-added products and solutions for production and companion animals. In 2018, ADM purchased Probiotics International Ltd., a provider of probiotic supplements for human, pet and production animal use and in 2017, it bought Crosswind Industries, Inc., a producer of dry-expanded, dual-texture, semi-dry and semi-moist treat products for pets.



